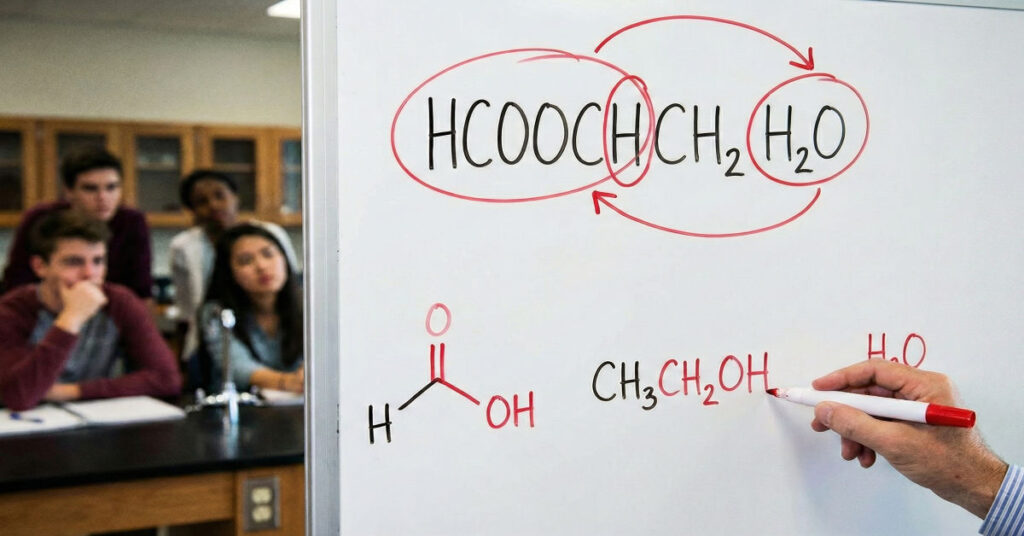
Chemists encountering the formula “HCOOCH CH₂ H₂O” immediately notice that it cannot represent a valid molecule. The sequence appears to blend an ester fragment, a methylene group, and water into a structure that violates basic bonding rules. Still, beneath this confusion lie real and important chemical concepts. Clarifying them provides not only a useful lesson in molecular notation but also a window into ester chemistry and the industrial processes that rely on it.
So here it is plainly “HCOOCH CH₂ H₂O” is not a real compound. It is a miswritten mixture of fragments—HCOOCH (an ester component), CH₂ (a methylene or vinyl fragment), and H₂O (water). No official chemical registry or standard nomenclature system recognizes such a structure.
Despite its inaccuracy, the miswritten formula mirrors two legitimate chemical scenarios: one involving methyl formate and water, and another involving vinyl formate in aqueous conditions. Both scenarios involve real molecules, real industrial processes, and real reactivity. Understanding these helps illuminate why correct notation matters for safety, research, and communication.
Why the Formula Is Chemically Impossible
At first glance, the string “HCOOCH CH₂ H₂O” lacks the connectivity required for a stable, covalently bonded structure. It resembles disconnected fragments more than a coherent molecule:
- HCOOCH resembles part of an ester structure.
- CH₂ could imply a methylene group or part of a vinyl linkage.
- H₂O is simply water.
Chemical formulas must indicate molecular identity, not merely list fragments. Without defined bonds, order, or stoichiometric ratios, the notation becomes ambiguous. Chemists rely on standard conventions because the difference between a fragment and a molecule can determine physical properties, reactivity, and safety protocols.
Moreover, no known synthesis produces a single, stable molecule composed of these fragments in this arrangement. Instead, the expression seems to arise from misunderstandings of ester notation or from attempts to compress a reaction description into an invalid molecular formula.
The Real Chemistry the Notation Likely Refers To
A closer look suggests two credible interpretations:
- Methyl formate reacting with water.
- Vinyl formate under aqueous conditions.
Interpretation 1: Methyl Formate and Water (Ester Hydrolysis)
One likely origin of the mistaken formula is methyl formate, properly written as HCOOCH₃, which is a well-defined ester. When methyl formate encounters water under acidic or basic conditions, it undergoes hydrolysis, producing formic acid and methanol.
The reaction is:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This transformation is widely used in industrial chemistry. Formic acid plays a role in leather production, textile processing, agricultural applications, and chemical synthesis. Methyl formate serves as an efficient precursor because hydrolysis conditions can be tuned to produce high yields of formic acid while recovering useful methanol as a byproduct.
Thus, the flawed notation “HCOOCH CH₂ H₂O” may stem from someone trying to reference methyl formate (HCOOCH₃) but accidentally omitting the final hydrogen, inserting a stray CH₂, or blending reaction components into a single pseudo-formula.
Vinyl Formate and Water (Hydrolysis and Tautomerization)
Another interpretation treats “HCOOCH CH₂” as shorthand for vinyl formate, the ester of formic acid and vinyl alcohol. Its correct structural form is HCOOCH=CH₂. Vinyl formate is real, structurally stable, and recognized in organic chemistry literature.
Like other formate esters, vinyl formate can hydrolyze in aqueous conditions. The products differ slightly from the methyl formate case:
- Hydrolysis yields formic acid and vinyl alcohol.
- Vinyl alcohol is unstable and rapidly rearranges (tautomerizes) to acetaldehyde, a widely known industrial aldehyde.
Thus, the fragments in the erroneous formula might reflect the idea of vinyl formate interacting with water, though the notation remains chemically improper.
Comparison of the Two Likely Correct Interpretations
Below is a structured comparison of the two mainstream interpretations of the miswritten formula.
Possible Interpretations of “HCOOCH CH₂ H₂O”
| Interpretation | Correct Formula | Key Reaction or Context | Notes |
| Methyl formate + water | HCOOCH₃ + H₂O | Hydrolysis → formic acid + methanol | Industrially important; original formula likely dropped a hydrogen and added stray CH₂ |
| Vinyl formate in water | HCOOCH=CH₂ + H₂O | Hydrolysis → formic acid + vinyl alcohol (→ acetaldehyde) | Possible misreading of CH=CH₂ as CH₂ |
How the Misnotation Spread Across Informal Sources
In online spaces—forums, casual chemistry blogs, quick-reference sites—chemical notation often drifts away from standard rules. Several factors contribute to the persistence of incorrect formulas:
- Over-simplification: Writers attempting to make chemistry accessible may collapse complex notation into loose fragments.
- Typographical errors: Missing subscripts or misplaced units can transform a correct formula into a nonsensical one.
- Non-specialist authorship: Without training in structural chemistry, some writers treat fragments as interchangeable.
- Misunderstanding of reaction notation: Some may confuse “reactants written side-by-side” with a combined molecular formula.
In many cases, “HCOOCH CH₂ H₂O” appears to be an attempt at shorthand for reactants rather than a true molecular formula.
Why Correct Chemical Notation Matters
Even minor deviations in chemical notation can cause significant confusion or errors. Incorrect formulas may lead to:
- Misinterpretation of laboratory procedures and unsafe handling of materials.
- Faulty literature searches, since chemical databases rely on standardized naming.
- Miscommunication among researchers, impacting reproducibility.
- Educational misunderstandings, creating misconceptions among students or hobbyists.
Clear notation ensures that structural information, bonding, and functional groups are communicated accurately. In chemistry, precision is not elitism—it is necessity.
Industrial Importance of Methyl Formate Hydrolysis
Beyond theory, ester hydrolysis plays a central role in chemical manufacturing. The conversion of methyl formate to formic acid demonstrates how reaction conditions can be engineered to optimize yield and efficiency.
Industrial Context of Methyl Formate Hydrolysis
| Factor | Role in Process | Effect |
| Temperature (typically elevated) | Accelerates ester hydrolysis | Shorter reaction times, improved kinetics |
| Catalysts (acidic or basic) | Promote bond cleavage | Higher efficiency and controlled reaction pathways |
| Distillation integration | Separates methanol and formic acid | Enables continuous production |
| Pressure adjustments | Stabilize reaction environment | Maintains desired phase behavior |
These considerations make methyl formate hydrolysis a preferred route for large-scale formic acid production. The reaction is relatively clean, avoids harsher oxidation processes, and produces a valuable alcohol byproduct.
Why This Miswritten Formula Still Matters
The very existence of an incorrect formula circulating online reveals something important: chemical literacy remains uneven, and people often rely on poorly curated information when learning or explaining scientific concepts.
Yet correcting these misunderstandings offers a chance to reinforce fundamental principles:
- Molecular formulas must reflect actual bonding.
- Fragments are not interchangeable with molecules.
- Reaction mixtures should be written as reactants, not compressed into one string.
Understanding the real chemistry behind the mistake—esters, hydrolysis, formates—turns a confusion into a learning opportunity.
Takeaways
- “HCOOCH CH₂ H₂O” does not represent any real chemical compound.
- The notation likely attempts to reference methyl formate with water or vinyl formate in aqueous conditions.
- Methyl formate hydrolysis is a real, industrially important process producing formic acid and methanol.
- Vinyl formate is a legitimate compound, but the miswritten formula does not correctly depict it.
- Accurate chemical notation is essential for safety, communication, and scientific reliability.
Conclusion
Chemical notation is more than symbolic shorthand it is a precise language. When that language breaks down, misunderstandings multiply. The formula “HCOOCH CH₂ H₂O” is not a real molecule, yet its fragments point toward meaningful chemistry—esters, hydrolysis, formic acid production, and the structural nuances that define organic compounds.
By tracing how a miswritten formula emerges and what real chemistry it echoes, we gain insight not only into molecular structures but also into the importance of clarity in scientific communication. Chemistry thrives on precision. Whether in a research lab an industrial facility, or a textbook, every letter, subscript and bond matters.
FAQs
1. Is “HCOOCH CH₂ H₂O” a valid molecular formula?
No. It is not recognized as a real chemical compound and lacks coherent bonding or structure.
2. What correct compound might the writer have intended?
Most likely methyl formate (HCOOCH₃) or vinyl formate (HCOOCH=CH₂) in the presence of water.
3. Why might someone miswrite the formula?
Common causes include typographical errors, confusion between fragments and whole molecules, or attempts to compress reactants into a single expression.
4. What happens when methyl formate reacts with water?
It undergoes hydrolysis, forming formic acid and methanol under suitable conditions.
5. Why is correct chemical notation important?
It ensures safety, reproducibility, accurate communication, and proper understanding of molecular behavior.
References
Formic Acid – Industrial Production & Chemical Behavior
National Center for Biotechnology Information. (2023). Formic acid. PubChem Compound Summary for CID 284.
https://pubchem.ncbi.nlm.nih.gov/compound/Formic-acid
Vinyl Formate – Chemical Properties
National Center for Biotechnology Information. (2023). Vinyl formate. PubChem Compound Summary for CID 10771.
https://pubchem.ncbi.nlm.nih.gov/compound/Vinyl-formate
Ester Reactions and Nomenclature – IUPAC Standards
International Union of Pure and Applied Chemistry (IUPAC). (2014). Nomenclature of organic chemistry: IUPAC recommendations and preferred names 2013. Royal Society of Chemistry.
https://iupac.qmul.ac.uk/BlueBook/
Industrial Hydrolysis Processes – Chemical Engineering Basis
Ullmann’s Encyclopedia of Industrial Chemistry. (2020). Formic Acid. Wiley-VCH.
https://onlinelibrary.wiley.com/doi/book/10.1002/14356007
Acetaldehyde Formation from Vinyl Alcohol – Tautomerization Evidence
National Center for Biotechnology Information. (2023). Acetaldehyde. PubChem Compound Summary for CID 177.
https://pubchem.ncbi.nlm.nih.gov/compound/Acetaldehyde